
Marine Research


Suspension of disbelief:
Deep-sea animals continue to defy conventional ideas about nutrition


click here for a dive into the deep sea
Symbiotic partnerships involving two or more species are widespread in nature and we can expect to find them in every type of environment, from rainforests, to urban landscapes, to the oceans. It is clear that microbes are supremely important to the success of most animals, including humans, and that many dietary strategies would not be possible were it not for intimately associated bacteria. Blood-feeding marine invertebrates are no exception in that they contend with numerous obstacles associated with feeding on the blood of fish and sharks – a difficult to breakdown, toxic, and nutritionally deficient diet. Bacteria in the digestive systems of nearly all animals examined play critical roles in the exchange of nutrients and digestion of food, and thus contribute to success and survival of the host, yet virtually nothing is known about the influence of internal bacteria on the immense success of blood-feeding marine invertebrates. Blood-feeding animals are not only important to study because of their potential symbiotic relationships with microbes, but because of their ability to act as both vectors for pathogens and the harm they cause to fish stocks. The proposed research combines a variety of molecular, imaging, and experimental approaches to examine whether internal bacteria positively influence the success of this unusual group of marine parasites. Integrated with this proposal are research opportunities for undergraduates, and the expansion of a college-level course that incorporates active exploration, including contemporary molecular and imaging techniques.
Click Goffredi_EM_2012.pdf for our 2012 publication on deep-sea leeches
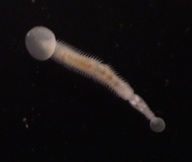
Marine polychaetes of the genus Osedax are involved in an unusual symbiosis with intracellular bacteria belonging to the order Oceanospirillales. Ecologically, these worms and their microbial symbionts play a substantial role in the cycling of carbon from deep-sea whalefalls. Temporal variation in the relative abundances of microheterogeneous ribotypes exists among the Osedax symbionts. For example, in worms at depths between 1800-3000 m, disproportionately greater numbers of symbiont ribotype Rs2 occur at later points in time (> 36 months), as compared to Rs1, which is nearly exclusive in its occurrence at the early stages of a whalefall. This shift occurs independently of shifts in host species composition, resulting perhaps from local adaptations to temporal changes in environmental gradients encountered by the pre-infectious "free-living" bacteria. The draft genomes of these two dominant symbionts were sequenced and reveal heterotrophic versatility in carbon uptake, strategies for intracellular survival, evidence for an independent existence, and potential virulence capabilities. Notably, large genome sizes (~ 4.6Mb) and presence of both flagellin and chemotaxis-related genes supports the assumption of an environmental pool of symbionts from which newly-settled Osedax larvae form associations. Environmental symbionts must face conflicting selective pressures for their host-associated and free-living subpopulations. Observed genomic differences in carbon and sulfur metabolism between Rs1 and Rs2 are consistent with the hypothesis that the environment controls the symbiont populations at a given time, and that hosts primarily acquire the locally-adapted symbiont. For example, the late-arriving Rs2 symbiont uniquely carries genes for sulfide oxidation (soxABDXYZ), suggesting the capacity to detoxify sulfide, which is observed to accumulate in the environment at later stages of whalefall decomposition. Direct influence on symbiont populations by dynamic changes in the local environment is likely for all marine symbioses where the host initially encounters symbionts in the water column or from substrate.
Click Worms close-up copy.mov to see a movie of Osedax (taken via submersible, MBARI (c))
Click Goffredi_2014_ISMEJ.pdf for our publication on Osedax symbionts
Click Rouse_2018.pdf for our publication on 14 new Osedax species
For several years, the hairy yeti crab, Kiwa hirsuta, was the only deep-sea member of the recently discovered deep-sea crab family Kiwaidae. Although this species was first found in a remote area of active hydrothermal venting along the Pacific Antarctic Ridge in 2005, another population was discovered just off the west coast of Costa Rica in 2008. The yeti crabs are unique because of their relationship with epibiotic bacteria, which cover most of their appendages including chelipeds and walking legs. Recently, the ultrastructure of the PAR K. hirsuta symbiosis and the phylogenetic and functional characterization of the epibiotic bacterial assemblage were investigated. Despite this previous data, there are still several important questions that remain. We study the symbiosis between the Costa Rican yeti crab and its epibionts, also known to consist of at least 3-4 dominant types of bacteria. Using molecular biology and microscopy, we compare and contrast the PAR and Costa Rica populations (that live ~4000 km from each other), as well as look at the life stage at which these crabs acquire their bacteria and if it occurs all at once or over time.
Click Goffredi_2014_MEC.pdf for our publication on the symbionts of baby yeti crabs

Comparative ‘omics of the bacterial symbionts in Osedax
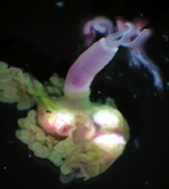
Nutritional Symbiosis between bacteria and hematophagous animals
Symbiosis of the Hairy Yeti Crab (Kiwa hirsuta) and Epibiotic Bacteria


We have discovered two deep-sea annelid species from Costa Rica that have independently established highly-specific, persistent, nutritional symbioses with aerobic methanotrophic bacteria. Unexpectedly, rather than suspension feeding, which is the nutritional strategy of all other known serpulids and sabellids, these worms host bacteria externally, and engulf them via their epidermal surfaces. In addition, by using both autonomous underwater vehicle (AUV) surveys and human occupied vehicle (HOV) dives, the discovery of these new symbioses illustrates an expansion of the traditional criteria for methane seep ecosystems, and brings us one step closer to understanding the spatial influence of seepage to guide regulatory actions along the world’s active continental margins. Similarly, in 2018, we discovered large populations of deep-sea anemones by submersible in the Gulf of California, on the deepest known hydrothermal vents in the Pacific Ocean, at a depth of about 3800 meters. These deep-sea anemones were in high abundance, comparable to the nearby deep-sea tubeworms (Oasisia alvinae) that were already known to rely on chemosynthesis, using sulfide from the vent for energy (Goffredi et al. 2017). We have preliminary evidence that this species of anemone is also chemosynthetic, relying on sulfide-oxidizing bacteria in it’s tentacles to thrive in this unique environment.
Click Goffredi et al_2021.pdf for our related publication
